Introduction
HMAs are the only approved therapy for CMML. In order to improve response rates and survival, the addition of VEN has been utilized in both CMML and after blast transformation (CMML-BT) with conflicting results in small single center studies. To clarify the role of upfront HMA+VEN in CMML and CMML-BT, we performed a large multicenter analysis.
Methods
We included patients with HMA-naïve CMML and CMML-BT treated with combination HMA and VEN from 4 academic centers. The primary outcome was overall survival (OS) with secondary outcomes including overall response rate (ORR) by 2015 MDS/MPN IWG criteria for CMML and 2022 ELN criteria for CMML-BT.
To better appreciate the benefit of adding VEN to HMA treatment, we also performed 1:1 propensity score matched (PSM) analysis of patients treated with HMA alone as upfront therapy for CMML or CMML-BT. Matching was based on age, sex, ECOG performance status, CPSS-Mol, blood counts, peripheral and bone marrow blasts.
Results
Eighty-nine patients were treated with frontline HMA+VEN, 51 CMML and 38 CMML-BT (5 had received HMA alone for prior CMML). The median age was 71 years, 70% were male, and 83% had ECOG 0-1. Among CMML patients, 55% had MP-CMML subtype, 65% had CMML-2 and 12%, 30%, 58% were CPSS-mol intermediate-1, intermediate-2 and high risk, respectively. In the entire cohort, ASXL1 (63%), TET2 (47%), SRSF2 (39%) and RUNX1 (27%) were the most commonly mutated genes and 55% of patients harbored a RAS mutation ( KRAS [20%], NRAS [18%], CBL [13%], NF1 [11%], PTPN11 [9%]). For HMA combination partner, 46 (52%) were treated with azacitidine, 35 (39%) with decitabine, and 8 (9%) with both during their VEN course. During a median follow-up time of 15.8 months (mos) 52 patients (58%) died: 23 (45%) with CMML and 29 (76%) with CMML-BT.
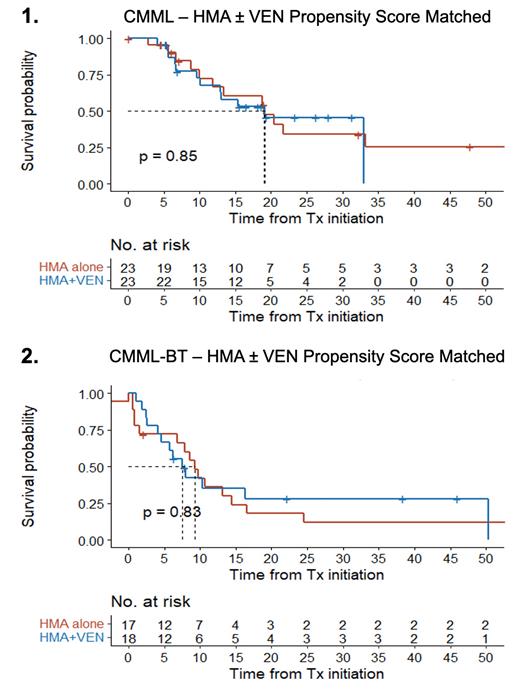
Among CMML patients, the ORR was 90%, including complete remission (CR) in 22%, complete cytogenic remission (CCR) in 4%, optimal marrow response (CMR) in 50%, and partial marrow response (PMR) in 6%. The OS of CMML patients was 25.1 mo (95% CI 15.7 - NR). A PSM population of HMA patients matched to HMA+VEN treated patients showing similar baseline characteristics. ORR was significantly higher with HMA+VEN (95%) as compared those treated with HMA alone (46%), p < 0.001. However, there was no significant OS difference between HMA+VEN vs HMA alone (19.1 mos vs 19.1 mos, p = 0.85, Figure 1).
For CMML-BT patients, the ORR of HMA+VEN was 81% including CR in 17%, CR with partial hematologic recover (CRh) in 3%, CR with incomplete hematologic recovery (CRi) in 28%, morphologic leukemia-free state (MLFS) in 22% and partial response (PR) in 11%. The OS of CMML-BT patients was 8 mos (95% CI 4.6 - 26.2). A PSM population of CMML-BT patients treated with HMA alone showed a significantly increased ORR with HMA+VEN (82% vs 40%, p = 0.039). However, there was no difference in OS (7.49 mos vs 9.26 mos, p = 0.83, Figure 2).
Among HMA-VEN treated patients, achievement of a CR was associated with an improved OS in the CMML (HR 0.31, 95% CI 0.13 - 0.75) and CMML-BT (HR 0.37, 95% CI 0.19 - 0.74). No predictors of response were identified, including the presence of a RAS mutation. In terms of safety, 7 patients (7.9%) had a grade 3/4 (G3/4) neutropenic infection and 2 patients (2.2%) had a G3/4 hemorrhagic event while being treated with HMA+VEN.
A total of 29 patients went on to receive allogeneic transplant (alloSCT): 22 (43%) with CMML and 7 (18%) with CMML-BT. CMML patients who underwent alloSCT after HMA+VEN were younger as compared to non-transplanted patients (64 vs 74 years, p < 0.0001), but had otherwise similar baseline characteristics including stage, CPSS-mol category and prevalence of RAS mutations. Similarly, CMML-BT patients bridged to alloSCT were younger (66 vs 74 years, p < 0.002) and had a better ECOG performance status (p = 0.047). Of patients who received alloSCT, 10 patients (34%) died, 5 with CMML and 5 with CMML-BT. The median OS after alloSCT was 26.7 mo.
Conclusions
Although the use of VEN with HMA in the upfront treatment of CMML and AML secondary to CMML results in higher response rates compared to HMA alone, this combination does not improve survival based on this multi-center PSM analysis. The current sample size of CMML-BT may limit detection of OS differences between treatment groups. The role of VEN to reduce blasts and serve as a bridge to alloSCT, particularly after HMA failure, requires further investigation in larger studies in both the CMML and CMML-BT populations.
OffLabel Disclosure:
Tremblay:AbbVie: Consultancy; Sierra Oncology: Consultancy; GSK: Consultancy; Cogent Biosciences: Consultancy; Astellas Pharma: Research Funding; Gilead: Research Funding; Novartis: Consultancy; CTI Biopharma: Consultancy, Research Funding. DiNardo:AbbVie/Genentech: Honoraria; Astellas: Honoraria; Servier: Honoraria; Schrödinger: Consultancy; Takeda: Honoraria; Notable Labs: Honoraria; Novartis: Honoraria; BMS: Honoraria; Fogham: Honoraria; ImmuniOnc: Honoraria. Kadia:Cure: Speakers Bureau; Cellenkos Inc.: Research Funding; Celgene: Research Funding; Biologix, Cure, Hikma Pharmaceuticals: Speakers Bureau; Genzyme: Honoraria; Astellas Pharma Global Development: Research Funding; AstraZeneca: Research Funding; Jazz Pharmaceuticals, Pfizer, Pulmotect, Inc, Regeneron Pharmaceuticals, SELLAS Life Sciences Group: Research Funding; Hikma Pharmaceuticals: Speakers Bureau; Pulmotect, Inc.: Consultancy, Research Funding; GenFleet Therapeutics: Research Funding; SELLAS Life Sciences Group: Research Funding; Pfizer: Consultancy, Research Funding; Delta-Fly Pharma, Inc.: Research Funding; Sanofi-Aventis: Consultancy; Amgen, Inc.: Research Funding; AbbVie, Amgen, Inc, Ascentage Pharma Group, Astellas Pharma Global Development, Astex, AstraZeneca, BMS, Celgene, Cellenkos Inc, Cyclacel, Delta-Fly Pharma, Inc, Genentech, Inc., Genfleet, Glycomimetics, Iterion, Janssen Research and Development: Research Funding; Cyclacel: Research Funding; Liberum: Consultancy; Glycomimetics: Research Funding; Genentech: Consultancy, Research Funding; Iterion: Research Funding; Janssen Research and Development: Research Funding; Novartis: Consultancy; Ascentage Pharma Group: Research Funding; Regeneron Pharmaceuticals: Research Funding; Daiichi Sankyo, Genentech, Inc., Genzyme, Jazz Pharmaceuticals, Liberum, Novartis, Pfizer, PinotBio, Inc, Pulmotect, Inc, Sanofi-Aventis, Servier: Consultancy; Agios: Consultancy; Servier: Consultancy; Pinotb-Bio: Consultancy; BMS: Consultancy, Research Funding; Astex: Honoraria. Ravandi:Astellas: Consultancy, Honoraria, Research Funding; Biomea fusion: Honoraria, Research Funding; Astex/taiho: Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene/BMS: Consultancy, Honoraria, Research Funding; Abbvie: Consultancy, Honoraria, Research Funding; Amgen: Honoraria, Research Funding; Xencor: Research Funding; Syros: Consultancy, Honoraria, Research Funding; Prelude: Research Funding. Chien:Rigel Pharmaceuticals: Consultancy; AbbVie: Consultancy. Kremyanskaya:Protagonist Therapeutics, Inc.: Consultancy, Research Funding. Feld:Gilead: Consultancy; Syros, Taiho, Oryzon: Research Funding. Komrokji:Novartis: Membership on an entity's Board of Directors or advisory committees; Geron: Consultancy; BMS: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Rigel, Taiho, DSI: Honoraria, Membership on an entity's Board of Directors or advisory committees; AbbVie, CTI biopharma, Jazz, Pharma Essentia, Servio: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Mascarenhas:Bristol Myers Squibb, Celgene, Constellation Pharmaceuticals/MorphoSys, CTI BioPharma, Galecto, Geron, GSK, Incyte Corporation, Karyopharm Therapeutics, Novartis, PharmaEssentia, Prelude Therapeutics, Pfizer, Merck, Roche, AbbVie, Kartos: Consultancy, Membership on an entity's Board of Directors or advisory committees; Bristol Myers Squibb, Celgene, CTI BioPharma, Geron, Incyte Corporation, Janssen, Kartos Therapeutics, Merck, Novartis, PharmaEssentia, Roche; Participated in consulting or advisory committees - AbbVie, Bristol Myers Squibb, Celgene, Constellation Pharmac: Research Funding; Incyte, Novartis, Roche, Geron, GSK, Celgene/BMS, Kartos, AbbVie, Karyopharm, PharmaEssentia, Galecto, Imago, Sierra Oncology, Pfizer, MorphoSys, CTI Bio: Consultancy; AbbVie, Bristol Myers Squibb, Celgene, CTI BioPharma, Geron, Incyte Corporation, Novartis, Janssen, Kartos Therapeutics, Merck, PharmaEssentia, Roche: Research Funding; GSK: Honoraria; AbbVie, CTI BioPharma Corp, a Sobi company, Geron, GlaxoSmithKline, Imago, Incyte, Kartos, Kayropharm, MorphoSys, Novartis, Pfizer, PharmaEssentia, Sierra: Consultancy. Padron:Gillead: Membership on an entity's Board of Directors or advisory committees; Abbvie: Membership on an entity's Board of Directors or advisory committees; Pharmaessentia: Membership on an entity's Board of Directors or advisory committees; CTI: Membership on an entity's Board of Directors or advisory committees; BMS: Research Funding; Incyte: Research Funding; Kura: Research Funding. Garcia-Manero:Bristol Myers Squibb: Other: Medical writing support, Research Funding; Genentech: Research Funding; AbbVie: Research Funding. Sallman:AbbVie, Affimed Gmbh, Gilead, Incyte, Intellisphere, LLC, Molecular Partners AG, PGEN Therapeutics, Inc., Takeda, Zentalis; Advisory board for AvenCell, BlueBird Bio, BMS, Intellia, Jasper Therapeutics, Kite, Magenta Therapeutics, NKARTA, Novartis, Orbita: Consultancy; Aprea, Jazz: Research Funding. Patnaik:Epigenetix: Research Funding; Kura: Research Funding; CTI BioPharma: Membership on an entity's Board of Directors or advisory committees; StemLine: Research Funding. Montalban-Bravo:Takeda: Research Funding; Rigel: Research Funding.
Venetoclax for CMML

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal